Roche's CD20xCD3 dual anti-mosunetuzumab obtained FDA breakthrough therapy designation
Recently (July 14), Roche announced that its CD20xCD3 T cell combined with dual-characteristic cancer immunotherapy mosunetuzumab has received FDA Breakthrough Therapy Designation (BTD).
Dr. Levi Garraway, Roche’s Chief Medical Officer and Head of Global Product Development, said: “We are very pleased that the FDA has granted mosunetuzumab breakthrough therapy designation and recognized the early efficacy data of this molecule.
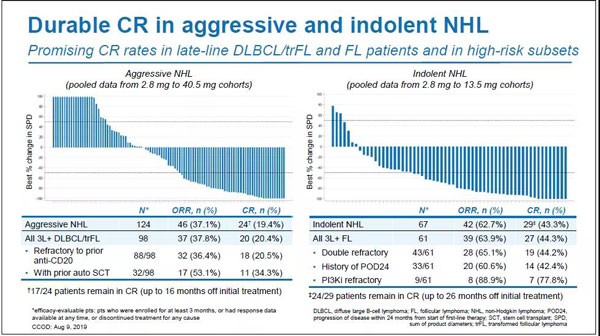
The early efficacy data mentioned here comes from a multi-center phase I/IIb clinical study code-named GO29781 (NCT02500407). At the 2019 ASH Conference, Roche announced the clinical data of the study. The results showed that for patients with refractory/relapsed non-Hodgkin's lymphoma who had received at least five lines (median) systemic treatment in advance (most of them did not respond to CD20 therapy, some relapsed after receiving CAR-T therapy) The effect of mosunetuzumab is remarkable.
In terms of objective response rate (ORR), inert NHL was 62.7% (n=42/67), aggressive NHL was 37.1% (n=46/124), and in terms of complete response rate (CR), inert NHL was 43.3% (n =29/67), invasive NHL was 19.4% (n=24/124). In terms of CR persistence, 82.8% (n=24/29) of indolent NHL patients were still in remission within 26 months after initial treatment, and 70.8% (n=17/24) of aggressive NHL patients were in remission after initial treatment16 It was still in remission within months.
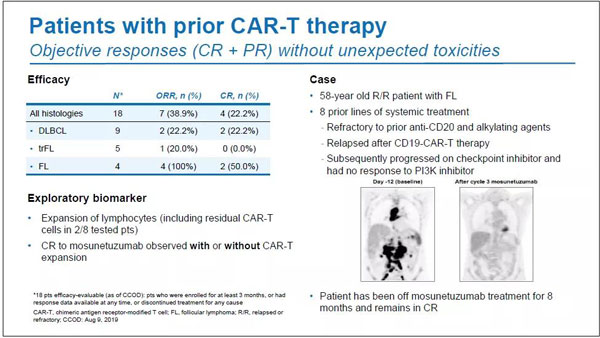
In the subgroup who received CAR-T therapy in advance, ORR and CR were 38.9% (n=7/18) and 22.2% (n=4/18), respectively.
In terms of safety, 28.9% of patients had cytokine release syndrome (CRS), of which 20.0% were grade 1, 1.1% were grade 3, and the incidence of grade 3 neurological adverse events was 3.7%.
Mosunetuzumab
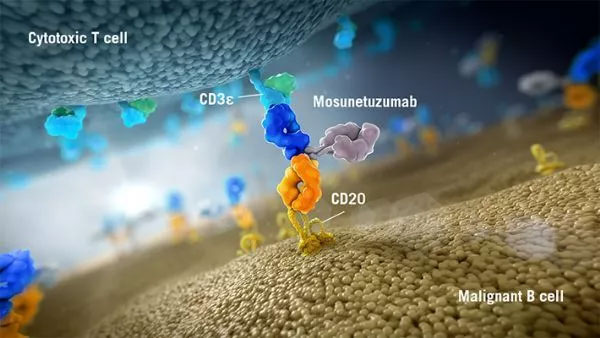
Mosunetuzumab is a bispecific antibody under development that can target CD20 on the surface of B cells and CD3 on the surface of T cells. This dual-targeting feature can activate and redirect patient T cells, contact and release cytotoxic proteins to B cells.
The structure of Mosunetuzumab is similar to human natural antibody, with two Fab segments. But unlike natural antibodies, one of the Fab targets CD20, while the other targets CD3. Currently, mosunetuzumab clinical research and development program is underway, its purpose is to explore the molecule as a single or combination therapy for patients with CD20-positive B-cell non-Hodgkin lymphoma (including follicular lymphoma, diffuse large B-cell lymphoma and other Blood cancer).
Other related
At present, Roche has obvious advantages in the field of CD20 target double antibodies. In addition to Mosunetuzumab, another CD20xCD3 bispecific antibody from Roche called Glofitamab is even more special. The antibody has a 2:1 TCB structure, including 2 anti-CD20 Fab and 1 anti-CD3 Fab.
The advantage of this structure is to help the formation of immune synapses. Its anti-CD3 and anti-TAA are only separated by CH1 and CL, which not only ensures sufficient flexibility, but also ensures that the overall size is within 15nM, ensuring the effectiveness of immune synapses. form. The formation of immune synapses directly affects the anti-tumor activity of the CD3/TAA double antibody. Under physiological conditions, the TCR and MHC-polypeptide are combined, and the distance between T cells and tumor cells is about 15nm. If the CD3/TAA double antibody, TAA is too large or the double antibody part is too large, it is easy to cause the distance between T cells and tumor cells to be greater than 15nm, which is not conducive to the formation of immune synapses. (For details, please click: Roche TriFab-Contorsbody: Promote immune synapse formation)
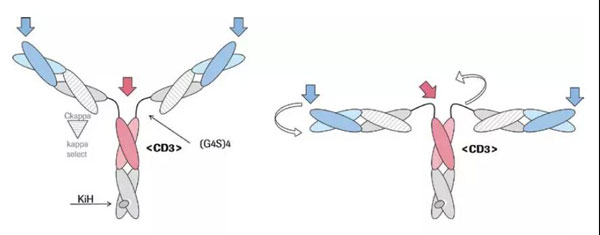
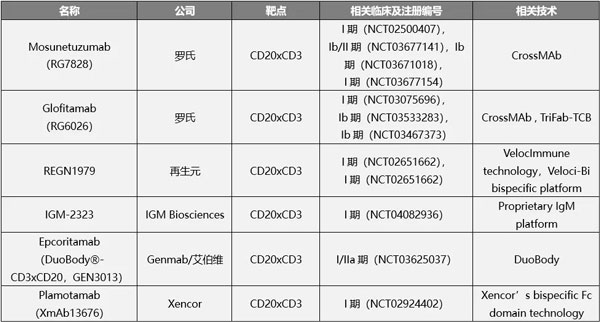
In addition to Roche, Regeneron, IGM Biosciences, Genmab, Xencor and other companies all have CD20xCD3 double antibody layouts. In China, Cinda and Roche have reached a US$2 billion cooperation, which includes TCB double antibodies, while Zai Lab introduced the regenerant CD20xCD3 bispecific antibody REGN1979 for US$190 million.
Research CD20xCD3 double antibody