The world's first BCMA targeted drug is really coming, and the FDA expert team supports the launch of GlaxoSmithKline ADC drug at 12:0
Last week, during the review process of GlaxoSmithKline’s targeted BCMA-ADC (antibody-drug conjugate)---Belantamab mafodotin, FDA staff expressed concern about the ocular toxicity and side effects of the drug treatment, although Ge Lan Su Shi Ke stated that no patients have permanent loss of vision, and tried their best to ensure that the side effects are managed, including eye examinations, and dose adjustments according to the situation. However, FDA staff still expressed doubts, believing that the company does not yet have clear treatment measures that can alleviate the side effects.
Belantamab mafodotin was in a phase II clinical trial called DREAM-2, and 40% of patients receiving treatment had ocular toxicity side effects above grade 3.
The Oncologic Drug Advisory Committee obviously has a different view on this. Yesterday (July 14, 2020), the Oncologic Drug Advisory Committee (ODAC, Oncologic Drug Advisory Committee) voted for Belantamab mafodotin with a 12:0 (for: against) vote. It is marketed for the fourth-line treatment of R/RMM patients who have previously received at least 3 treatment options (including lenalidomide, proteasome inhibitor and CD38 antibody).
ODAC’s recommendation is an important consideration in the FDA’s decision to approve the product’s listing, and ODAC’s unanimous statement can basically indicate that there is nothing suspenseful about the approval of this drug, and patients with multiple myeloma will finally usher in the world’s first product. BCMA is a targeted drug.
Belantamab mafodotin
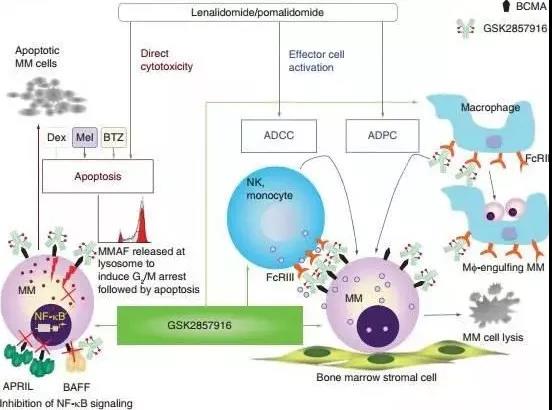
Belantamab mafodotin is an antibody-drug conjugate composed of monoclonal antibody and cytotoxic drug auristatin F through a non-cleavable linker. The linker technology of the drug is licensed from Seattle Genetices, and the monoclonal antibody is developed using BioWa technology. . The anti-BCMA monoclonal antibody partially targets multiple myeloma and releases MMAF after internalization to exert cytotoxicity. In addition, belantamab can also exert antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis ( ADCP).
Belantamab mafodotin has previously obtained FDA breakthrough drug and priority approval qualifications, and now it has also obtained two clinical approvals in China. The indication is combined with bortezomib and dexamethasone for the treatment of multiple bone marrow that has received at least one treatment in the past. Tumor (adult), the other is monotherapy.
On May 27, 2020, GlaxoSmithKline (GSK) announced the latest results of the DREAMM-2 and DREAMM-6 clinical trials of belantamab mafodotin for the treatment of R/R MM.
The results from the DREAMM-2 clinical study showed that at the 13-month follow-up, the median duration of response (DoR) of monotherapy was 11 months, and the median overall survival (OS) was 14.9 months. Among the patients who achieved remission, 58% achieved good partial remission, 2 cases of strict complete remission and 5 cases of complete remission. In the 2.5 mg/kg belantamab mafodotin dose group, the overall response rate (ORR) was consistent with the 6-month follow-up data (32%).
The results from the DREAMM-6 clinical study showed that the ORR of Belantamab Mafodotin+lenalidomide/bortezomib+dexamethasone (Arm A) in the treatment of patients with relapsed/refractory multiple myeloma reached 78%, and 50% reached very good Partial remission, 28% achieved partial remission.
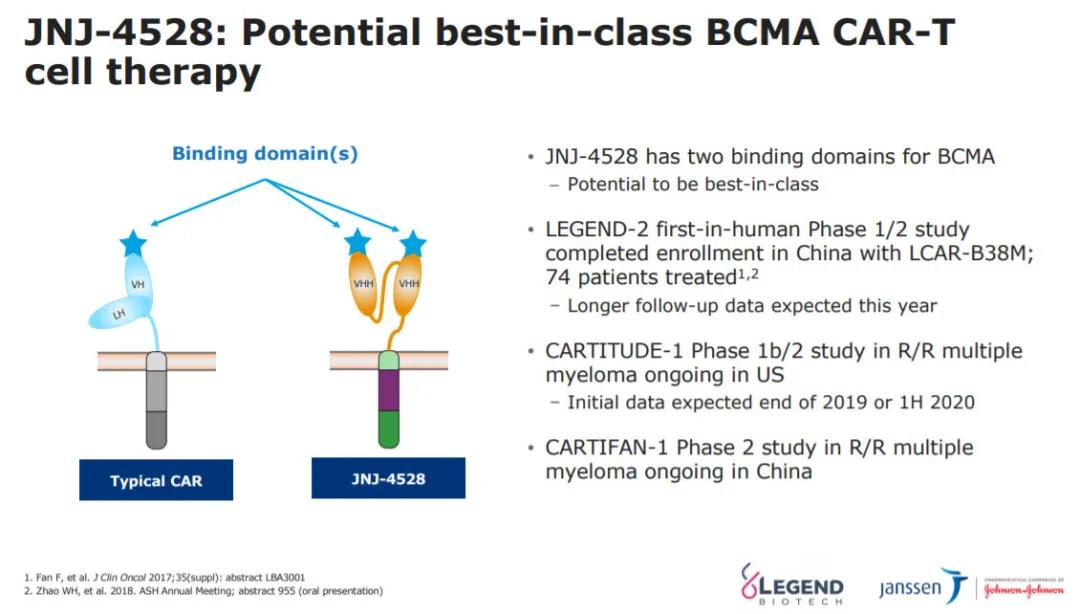
The market prospects of the BCMA target have stimulated the enthusiasm of the industry. Although belantamab mafodotin has the first-mover advantage to enter the market, strong opponents have already appeared. BMS/Bluebird’s BCMA-CAR-T therapy ---idecabtagene vicleucel (ide-cel, bb2121) entering the market is also a knock-on, idecabtagene vicleucel has been granted accelerated approval qualification by EMA, and the marketing authorization application (MAA) has been accepted by the European Medicines Agency (EMA). Johnson & Johnson/Legendary's JNJ-4528 is also fully catching up. The BLA of JNJ-4528 will be submitted to the FDA in the second half of the year for the treatment of relapsed/refractory multiple myeloma. On May 14, Johnson & Johnson/Legendary Biologics updated the clinical data of the CARTITUDE-1 study of JNJ-4528 for the treatment of r/r MM patients. The clinical data is still very impressive. At a median follow-up time of 11.5 months, the total remission rate was 100. %, the complete remission rate increased from 69% announced 5 months ago to 86%. Of the 29 patients, 22 had progression-free survival.
The field of double antibodies is also ready to move. Amgen's bispecific antibody targeting BCMA/CD3, AMG420, has been granted the status of fast-track approval by the FDA. In June last year, Amgen announced the latest data from the Phase I clinical trial at the American Society of Clinical Oncology (ASCO) annual meeting. The results showed that among 42 R/RMM patients enrolled, the overall response rate (ORR) was 70%, the median duration of remission was 9 months.
Conclusion
Targeted drugs such as CD38 antibodies have greatly changed the treatment status of patients with multiple myeloma, but the widespread high recurrence rate of multiple myeloma is still very difficult to manage. BCMA targeted therapy is effective for recurrence after CD38 treatment. Drug-resistant patients can also show therapeutic activity, opening a window for the solution of recurring problems.
Belantamab Mafodotin has won a lot of money in this field, and CAR-T therapy, monoclonal antibodies, and double antibodies targeting BCMA are not succumbing to lagging behind. As soon as Belantamab Mafodotin is listed, it will face CAR-T, a strong opponent. From the current efficacy and safety of the two, Belantamab Mafodotin is inferior to CAR-T, but the former is better in terms of accessibility (difficulty of production and treatment costs), but in the end, for patients, the treatment plan is of course the more the better.