Heavy! BeiGene's partner BioAtla completes US$72.5 million in Series D financing
On July 15, 2020, San Diego BioAtla, Inc. (BioAtla) is a global clinical-stage biotechnology company dedicated to the development of conditional activated biological (CAB) antibody therapies. It announced the completion of the D round of financing and raised 72.5 million US dollars. . The financing was led by Soleus Capital and several new investors joined, including HBM Healthcare Investments as co-leader, Cormorant Asset Management, Farallon Capital, Pappas Capital, funds managed by Janus Henderson, Boxer Capital and other institutional investors. Pfizer Ventures, an existing investor in Pfizer's venture capital arm, also participated in the financing. The company has previously raised more than 114 million U.S. dollars and is expected to submit an IPO soon.
Said Dr. Jay M. Short, Chairman and CEO of BioAtla. "The funds provided by this highly respected group of investors strongly support the implementation of BioAtla's current and future products and strategic plans. The proceeds of this financing have greatly enhanced our design, implementation and execution from our unique CAB platform. The ability of the coming clinical project. It can produce tumor-targeting antibodies, which has the potential to increase the benefit and risk."
Scott Smith, President of BioAtla, said, "We look forward to promoting Phase 2 clinical trials for the innovative CAB-AXL-ADC (BA3011) and CAB-ROR2-ADC (BA3021) product plans to meet the highly unmet medical needs of oncology, and to advance CAB- A clinical study of CTLA-4 (BA3071). In addition, we are developing several CAB bispecific drug candidates that recruit T cells."

On December 7, 2016, BioAtla, LLC announced that it and Peking University Weiming Bioengineering Group Co., Ltd. ("Weiming Group") have selected the first batch of four CAB product projects jointly developed by the two parties. The specific candidate projects and the targeted indications were not disclosed. In March 2015, BioAtla (BioAtla) and Weiming Group reached a strategic cooperation on the commercial development of specific CAB antibodies for specific indications in China, Hong Kong, Macau and Taiwan ("China"). Therefore, according to the cooperation agreement, after selecting all four 2016 CAB drug candidates for development, BioAtla received a payment of 36 million U.S. dollars from the Weiming Group on November 1, 2016, and the money was a joint development institute between the two parties. The remaining funds for the four selected 2016 CAB drug candidates (for specific indications). Including the above funds, BioAtla has received more than US$100 million in project payments and equity investments related to the signing of a strategic cooperation agreement with Weiming Group.

On April 9, 2019, BioAtla and BeiGene announced that they have reached a global joint development and cooperation agreement on the development, production and commercialization of BioAtla's CAB CTLA-4 antibody BA3071. BA3071 is a new type of CTLA-4 inhibitor, which can be conditioned in the tumor microenvironment to reduce systemic toxicity, so that it can interact with checkpoint inhibitors (such as BeiGene's tislelizumab, a design Human-derived IgG4 anti-PD-1 monoclonal antibody (anti-PD-1 monoclonal antibody) designed to avoid binding to Fc receptors in macrophages becomes a potentially safer combination therapy when combined with drugs.
According to the terms of the cooperation, BioAtla and BeiGene will jointly develop the CAB-CTLA-4 antibody to determine its early clinical goals, and then BeiGene will lead the joint clinical development of the candidate drug and be responsible for the global drug regulatory registration And commercialization. Subject to the terms of the agreement, BeiGene and BioAtla will share the exclusive global authorization for the development and production of drug candidates, and BeiGene has the exclusive authorization for the global commercialization of drug candidates. BeiGene will be responsible for all costs related to the development, production and commercialization of the candidate drug in Asia (except Japan), Australia and New Zealand; according to specific terms, the two parties will jointly bear the development and production related costs in countries and regions other than the above. And commercial profits and losses. BioAtla will receive a down payment of US$20 million, and a milestone payment will be made upon reaching established early clinical goals. In addition, BioAtla can also receive up to US$249 million for subsequent global development, pharmaceutical regulatory milestone payments, and commercialization milestone payments and sales tiered royalties in designated areas of BeiGene. Himalaya Therapeutics, a majority-owned subsidiary of BioAtla, will also receive the aforementioned milestone payments and royalties due to its participation in the development and commercialization in China.

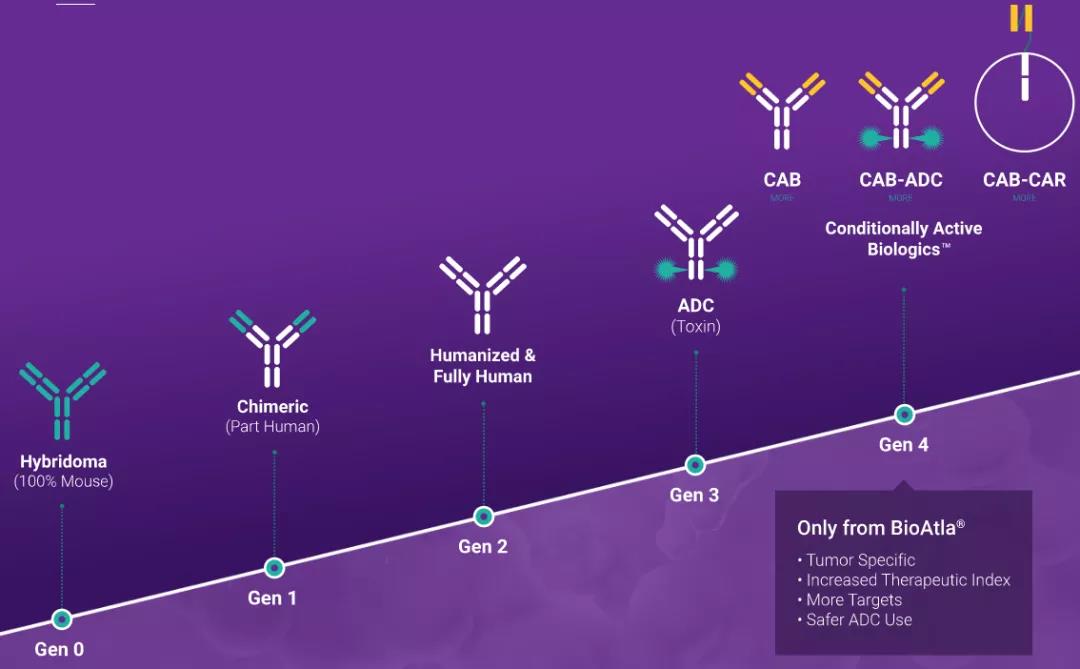
About Conditional Activated Biological Agents (CAB)
Conditionally activated biologics are proteins produced using BioAtla's proprietary protein discovery, evolution and expression technology. These proteins can be monoclonal antibodies, enzymes and other proteins, and their function depends on the micro-physiological conditions outside and inside the cell (for example, pH level, oxidation, temperature, pressure, the presence of certain ions, hydrophobicity and combinations thereof) Variety.
Studies have shown that cancerous tumors produce highly specific conditions at their sites, and this condition does not exist in normal tissues. These cancerous microenvironments are mainly the result of the well-known unique glycolytic metabolism associated with cancer cells, which is called the Warburg effect in aerobic cancer cells. CAB protein is designed to deliver its therapeutic payload and/or recruit immune responses under specific and selected locations and conditions in the body, and is only active in the presence of specific cellular microenvironments. In addition, activation is designed to be reversible to repeatedly "open and close" when the CAB changes from a diseased microenvironment to a normal cellular microenvironment, and vice versa. CAB can be developed in many forms, including antibodies, antibody-drug conjugates (ADC), bispecific antibodies, chimeric antigen receptor T cells (CAR-T) and combination therapies.
About BioAtla, Inc.
BioAtla is a global clinical stage biotechnology company with operations in San Diego, California and Beijing, China. Compared with traditional antibodies, BioAtla has developed new monoclonal antibodies and other protein therapeutic product candidates, which are designed to have higher selective targeting, higher efficacy, and more cost-effective and predictable production ability. BioAtla currently has two projects undergoing phase 1/2 clinical testing in the United States. BA3011 is a new type of conditionally activated AXL targeting antibody-drug conjugate (CAB-AXL-ADC), and BA3021 is a new type of conditionally activated ROR2 Targeting antibody-drug conjugate (CAB-ROR2-ADC). BioAtla's research CAB CTLA-4 antibody BA3071 has reached a global joint development and cooperation agreement with BeiGene Co., Ltd. on its development, production and commercialization. BA3071 is a new type of CTLA-4 inhibitor, designed to be conditionally activated in the tumor microenvironment to reduce systemic toxicity, and may be combined with checkpoint inhibitors (such as BeiGene's anti-PD-1 antibody, instead of Ralizumab) safe combination. 2019 Tmall Double 11 Carnival Night, Black Technology and Changeable Stage





